Boric acid
| Boric acid | |
|---|---|
 |
 |
 |
|
|
Boric acid
Trihydroxidoboron |
|
|
Other names
Orthoboric acid,
Boracic acid, Sassolite, Optibor, Borofax |
|
| Identifiers | |
| CAS number | 10043-35-3 |
| PubChem | 7628 |
| ChemSpider | 7346 |
|
SMILES
OB(O)O
|
|
|
InChI
InChI=1/BH3O3/c2-1(3)4/h2-4H
Key: KGBXLFKZBHKPEV-UHFFFAOYAI |
|
| Properties | |
| Molecular formula | H3BO3 |
| Molar mass | 61.83 g mol−1 |
| Appearance | White crystalline solid |
| Density | 1.435 g/cm3 |
| Melting point |
170.9 °C, 444 K, 340 °F |
| Boiling point |
300 °C, 573 K, 572 °F |
| Solubility in water | 2.52 g/100 mL (0 °C) 4.72 g/100 mL (20 °C) 5.7 g/100 mL (25°C) 19.10 g/100 mL (80 °C) 27.53 g/100 mL (100 °C) |
| Solubility in other solvents | Soluble in lower alcohols moderately soluble in pyridine very slightly soluble in acetone |
| Acidity (pKa) | 5.2 (see text) |
| Structure | |
| Molecular shape | Trigonal planar |
| Dipole moment | Zero |
| Hazards | |
| EU classification | Harmful (Xn) Repr. Cat. 2 |
| R-phrases | R60 R61 |
| S-phrases | S53 S45 |
| NFPA 704 |
 0
1
0
|
| Flash point | Non-flammable. |
| LD50 | 2660 mg/kg, oral (rat) |
| Related compounds | |
| Related compounds | Boron trioxide Borax |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Boric acid, also called boracic acid or orthoboric acid or acidum boricum, is a weak acid often used as an antiseptic, insecticide, flame retardant, in nuclear power plants to control the fission rate of uranium, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a white powder and dissolves in water. It has the chemical formula H3BO3, sometimes written B(OH)3. When occurring as a mineral, it is called sassolite.
Contents |
Occurrence
The free acid is found native in certain volcanic districts such as Tuscany, the Lipari Islands and Nevada, issuing mixed with steam from fissures in the ground; it is also found as a constituent of many minerals (borax, boracite, boronatrocaicite and colemanite). The presence of boric acid and its salts has been noted in seawater. It also exists in plants and especially in almost all fruits.[1]
Boric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name sal sedativum Hombergi ("sedative salt of Homberg"). However Borates, including boric acid, have been used since the time of the Greeks for cleaning, preserving food, and other activities.
Preparation
Boric acid may be prepared by reacting borax (sodium tetraborate decahydrate) with a mineral acid, such as hydrochloric acid:
- Na2B4O7·10H2O + 2 HCl → 4 B(OH)3 [or H3BO3] + 2 NaCl + 5 H2O
Properties
Boric acid is soluble in boiling water. When heated above 170 °C, it dehydrates, forming metaboric acid (HBO2):
- H3BO3 → HBO2 + H2O
Metaboric acid is a white, cubic crystalline solid and is only slightly soluble in water. Boric acid melts at about 236 °C, and when heated above about 300 °C further dehydrates, forming tetraboric acid or pyroboric acid (H2B4O7):
- 4 HBO2 → H2B4O7 + H2O
The term boric acid may sometimes refer to any of these compounds. Further heating leads to boron trioxide.
- H2B4O7 → 2 B2O3 + H2O
Boric acid does not dissociate in aqueous solution as a Brønsted acid, but is a Lewis acid which interacts with water molecules to form the tetrahydroxyborate ion, as observed by Raman spectroscopy[2]:
- B(OH)3 + H2O
 B(OH)−4 + H+ (Ka = 5.8x10−10 mol/l; pKa = 9.24)
B(OH)−4 + H+ (Ka = 5.8x10−10 mol/l; pKa = 9.24)
Polyborate anions are formed at pH 7–10 if the boron concentration is higher than about 0.025 mol/L. The best known of these is the tetraborate ion, found in the mineral borax:
- 4 B(OH)−4 + 2 H+ ⇌ B4O2−7 + 9 H2O
Boric acid makes an important contribution to the absorption of low frequency sound in seawater.[3]
Crystal structure
Crystalline boric acid consists of layers of B(OH)3 molecules held together by hydrogen bonds. The distance between two adjacent layers is 318 pm.
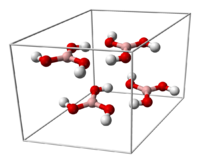 |
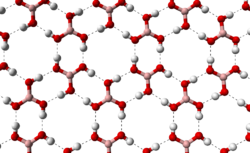 |
|
|
allows boric acid molecules to form parallel layers in the solid state |
Toxicology
Based on mammalian median lethal dose (LD50) rating of 2,660 mg/kg body mass, boric acid is poisonous if taken internally or inhaled in large quantities. However, it is generally considered to be not much more toxic than table salt.[4] The Thirteenth Edition of the Merck Index indicates that the LD50 of boric acid is 5.14 g/kg for oral dosages given to rats, and that 5 to 20 g/kg has produced death in adult humans. The LD50 of sodium chloride is reported to be 3.75 g/kg in rats according to the Merck Index.
Long term exposure to boric acid may be of more concern, causing kidney damage and eventually kidney failure (see links below). Although it does not appear to be carcinogenic, studies in dogs have reported testicular atrophy after exposure to 32 mg/kg bw/day for 90 days. This level is far lower than the LD50.[5]
According to boric acid IUCLID Dataset published by the European Commission, boric acid in high doses shows significant developmental toxicity and teratogenicity in rabbit, rat, and mouse fetuses as well as cardiovascular defects, skeletal variations, mild kidney lesions.[6] As a consequence, in August 2008, in the 30th ATP to EU directive 67/548/EEC, the EC decided to amend its classification as reprotoxic category 2 and to apply the risk phrases R60 (may impair fertility) and R61 (may cause harm to the unborn child).[7][8][9][10][11]
Uses
Medicinal
Boric acid can be used as an antiseptic for minor burns or cuts and is sometimes used in dressings or salves or is applied in a very dilute solution as an eye wash in a 1.5% solution (1 tbsp per quart or 15 cm3 per L) of sterilized water.
As an anti-bacterial compound, boric acid can also be used as an acne treatment. Boric acid can be used to treat yeast and fungal infections such as candidiasis (vaginal yeast infections) by inserting a vaginal suppository containing 600 mg of boric acid daily for 14 days[12] or for yeast infection of the male pubic region (jock-itch or strong genital odor) by applying the powder to the skin all over the pubic region for several days to a week. It is also used as prevention of athlete's foot, by inserting powder in the socks or stockings, and in solution can be used to treat some kinds of otitis externa (ear infection) in both humans and animals. The preservative in urine sample bottles (red cap) in the UK is boric acid.
TBE buffer is widely used for the electrophoresis of nucleic acids and has a higher buffer capacity than a TAE buffer. It can be used for DNA and RNA polyacrylamide and agarose gel electrophoresis.
Insecticidal
Boric acid was first registered in the US as an insecticide in 1948 for control of cockroaches, termites, fire ants, fleas, silverfish, and many other insects.[13] It acts as a stomach poison affecting the insects' metabolism, and the dry powder is abrasive to the insects' exoskeleton.
Boric acid is generally considered to be safe to use in household kitchens to control cockroaches and ants. Homemade ant bait can be made by dissolving 1 teaspoon powdered boric acid and 10 teaspoons sugar into 2 cups (~ 500 mL) of water; this mixture can then be absorbed into cotton balls which are left near ant trails. This reportedly will be carried back into the ants' nest, killing any ants that eat it, potentially destroying the entire colony[14].
Boric acid is also made into a paste or gel form as a powerful and effective insecticide much safer to humans than many other insecticides. The paste or gel has attractants in it to attract insects. The boric acid slowly causes dehydration.
Preservation
In combination with its use as an insecticide, boric acid also prevents and destroys existing wet and dry rot in timbers. It can be used in combination with an ethylene glycol carrier to treat external wood against fungal and insect attack. It is possible to buy borate-impregnated rods for insertion into wood via drill holes where dampness and moisture is known to collect and sit. It is available in a gel form and injectable paste form for treating rot affected wood without the need to replace the timber. Concentrates of borate-based treatments can be used to prevent slime, mycelium and algae growth, even in marine environments.
Boric acid is added to salt in the curing of cattle hides, calfskins and sheepskins. This helps to control bacteria development and helps to control insects.
Lubrication
Colloidal suspensions of nano-particles of boric acid dissolved in petroleum or vegetable oil can form a remarkable lubricant on ceramic or metal surfaces[15] with a coefficient of sliding friction that decreases with increasing pressure to a value ranging from 0.10 to 0.02. Self-lubricating H3BO3 films result from a spontaneous chemical reaction between water molecules and B2O3 coatings in a humid environment. In bulk-scale, an inverse relationship exists between friction coefficient and Hertzian contact pressure induced by applied load.
Industrial
The primary industrial use of boric acid is in the manufacture of monofilament fiberglass usually referred to as textile fiberglass. Textile fiberglass is used to reinforce plastics in applications that range from boats, to industrial piping to computer circuit boards.[16]
Boric acid is used in nuclear power plants as a neutron poison to slow down the rate at which fission is occurring. Fission chain reactions are generally driven by the amount of neutrons present (as products from previous fissions). Natural boron is 20% boron-10 and about 80% boron-11. Boron-10 has a high cross-section for absorption of low energy (thermal) neutrons. By adding more boric acid to the reactor coolant which circulates through the reactor, the probability that a neutron can survive to cause fission is reduced. Therefore, changes in boric acid concentration effectively regulate the rate of fission taking place in the reactor. This method is only used in pressurized water reactors (PWR's). Boron is also dissolved into the spent fuel pools containing used uranium rods. The concentration is high enough to keep neutron multiplication at a minimum.
In the jewelry industry, boric acid is often used in combination with denatured alcohol to reduce surface oxidation and firescale from forming on metals during annealing and soldering operations.
Boric acid is used the production of the glass in LCD flat panel displays.
In electroplating, boric acid is used as part of some proprietary formulas. One such known formula calls for about a 1 to 10 ratio of H3BO3 to NiSO4, a very small portion of sodium lauryl sulfate and a small portion of H2SO4.
It is also used in the manufacturing of ramming mass, a fine silica-containing powder used for producing induction furnace linings and ceramics.
Pyrotechnics
It is used in pyrotechnics to prevent the amide-forming reaction between aluminium and nitrates. A small amount of boric acid is added to the composition to neutralize alkaline amides that can react with the aluminium.
Boric acid can be used as a colorant to make fire green. For example, when dissolved in methanol it is popularly used among fire jugglers and fire spinners to create a deep green flame.
Other
Silly Putty was originally made by adding boric acid to silicone oil.
The white powder is also used to dust down Carrom boards to decrease friction and increase speed of play.[17]
Boric acid is added to borax for use as welding flux by blacksmiths and farriers.[18]
References
- ↑ A. H. Allen; Tankard, Arnold R. (1904). "The determination of boric acid in cider, fruits, etc". Analyst 29: 301. doi:10.1039/an9042900301.
- ↑ Jolly, William L. "Modern Inorganic Chemistry" (McGraw-Hill 1984, p.198)
- ↑ "Underlying physics and mechanisms for the absorption of sound in seawater". National Physical Laboratory. http://resource.npl.co.uk/acoustics/techguides/seaabsorption/physics.html. Retrieved 2008-04-21.
- ↑ F. Jay Murray (2004). "Don’t Lose Sleep Over Borates and Mattresses". Murray and Associates. http://web.archive.org/web/20070819100438/http://www.natbat.com/docs/boron.htm. Retrieved 2008-04-21.
- ↑ Office of Prevention, Pesticides and Toxic Substances (2006) (.PDF). Report of the Food Quality Protection Act (FQPA) Tolerance Reassessment Eligibility Decision (TRED) for Boric Acid/Sodium Borate Salts. United States Environmental Protection Agency. http://www.epa.gov/oppsrrd1/REDs/boric_acid_tred.pdf. Retrieved 2008-04-21.
- ↑ European Chemical Bureau - ECB (.PDF). Boric Acid IUCLID Dataset. European Commission. http://ecb.jrc.ec.europa.eu/IUCLID-DataSheets/10043353.pdf.
- ↑ "Boric acid, ACC# 03260". MSDS. 2008-02-11. http://lifesciences.byu.edu/safety/LabSafety/BoricAcid.pdf. Retrieved 2009-09-24.
- ↑ Ishii Y; Fujizuka N; Takahashi T; Shimizu K; Tuchida A; Yano S; Naruse T; Chishiro T. (1993). "A fatal case of acute boric acid poisoning". J Toxicol Clin Toxicol (Nagano, Japan) 31 (2): 345–352. doi:10.3109/15563659309000402. PMID 8492348. http://www.ncbi.nlm.nih.gov/pubmed/8492348.
- ↑ Restuccio, A.; Mortensen, M. E.; Kelley, M. T. (November 1992). "Fatal ingestion of boric acid in an adult". Am J Emerg Med 10 (6): 545–547. doi:10.1016/0735-6757(92)90180-6. PMID 1388380. http://www.find-health-articles.com/rec_pub_1388380-fatal-ingestion-boric-acid-adult.htm.
- ↑ John E. Duldner (2009-01-30). "Boric acid poisoning". A.D.A.M. Medical Encyclopedia. MedLine Plus. http://www.nlm.nih.gov/medlineplus/ency/article/002485.htm. Retrieved 2009-09-24.
- ↑ NSW Food Authority. "Borax and boric acid". Australia: New South Wales Government. http://www.foodauthority.nsw.gov.au/consumers/other-food-topics/borax-and-boric-acid/. Retrieved 2009-09-24.
- ↑ Ringdahl, EN (2000). "Treatment of recurrent vulvovaginal candidiasis.". American family physician 61 (11): 3306–12, 3317. PMID 10865926.
- ↑ Office of Prevention, Pesticides and Toxic Substances (1993) (.PDF). R.E.D. Facts: Boric Acid. United States Environmental Protection Agency. http://www.epa.gov/oppsrrd1/REDs/factsheets/0024fact.pdf. Retrieved 2008-04-21.
- ↑ "http://pest.tips.net/Pages/T003480 Use Acid and Poison to Make Homemade Ant Bait.html". April 2010. http://pest.tips.net/Pages/T003480_Use_Acid_and_Poison_to_Make_Homemade_Ant_Bait.html.
- ↑ H. Duumlzcuumlkogbrevelu ;M. Acarogbrevelu. "Lubrication Properties of Vegetable Oils Combined with Boric Acid and Determination of Their Effects on Wear". Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, Volume 32, Issue 3 January 2010 , pages 275 - 285. http://www.informaworld.com/smpp/content~content=a916410650~db=all~jumptype=rss. Retrieved 2010-03-23.
- ↑ R. B. Kistler; C. Helvaci (1983). Industrial Minerals and Rocks. Science Information Publishing Centre, Government of india's department for Scientific & Industrial Research
- ↑ Harpreet Singh. "Standard equipments". Punjab State Carrom Association. http://pscamohali.googlepages.com/standardequipments. Retrieved 2009-09-24.
- ↑ Jock Dempsey (1998, 2009). "BORAX". Dempsey's Forge. http://www.anvilfire.com/21centbs/material/borax01.htm. Retrieved 2010-07-23.
Further reading
- Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd Edn.). New York: McGraw-Hill. ISBN 0-07-112651-1.
- Louis Goodman, Alfred Gilman, Laurence Brunton, John Lazo and Keith Parker (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw Hill.
External links
- Boric Acid Technical Fact Sheet - National Pesticide Information Center
- Boric Acid General Fact Sheet - National Pesticide Information Center
- International Chemical Safety Card 0991
- US EPA Pesticide Reregistration Eligibility Decision
- National Pollutant Inventory - Boron and compounds
- Boric acid at ChemicalLand21
- European Chemicals Agency (ECHA)"New Public Consultation on Eight Potential Substances of Very High Concern" - includes Boric Acid. Closes 22nd April 2010
- ChemSub Online: Boric acid
|
||||||||||||||||||||